Available Facilities For Fertility Enhancement
Donor Oocyte Program
We have an ongoing program for Oocyte donation for women with premature ovarian failure.
The donors are physically, medically and genetically screened. This is one of the most comprehensive and reliable Donor Eggs and Donor Embryo Program in India.
 This oocyte donation program is also suitable for women with poor ovarian reserve, surgical or induced menopause and those in the perimenopausal group. Some women with repeated decreased recruitment of follicles at the time of IVF, poor quality of oocytes, high FSH, low AMH, with extensive endometriosis, severe pelvic adhesions and inaccessible ovaries can also avail of this facility.
This oocyte donation program is also suitable for women with poor ovarian reserve, surgical or induced menopause and those in the perimenopausal group. Some women with repeated decreased recruitment of follicles at the time of IVF, poor quality of oocytes, high FSH, low AMH, with extensive endometriosis, severe pelvic adhesions and inaccessible ovaries can also avail of this facility.
Egg donors are carefully screened and matched with recipients as closely as possible by physical characteristics. They are also screened for sexually transmitted diseases. In case of a genetic mutation in the male partner of the couple seeking Egg Donation, the Oocyte Donor is screened for a similar mutation so that the genetic disease is not passed on to the offspring. We ensure complete confidentiality and evaluate the background of the donors thoroughly. Donors also undergo a genetic test called Karyotyping in order to ensure that they have normal chromosomes. The family background of the donors is checked for genetic diseases. Advanced genetic testing is done as required. We follow ICMR guidelines for our Donor Egg Programme. Donors undergo whole exome analysis to look for any pathological variants, i.e. Gene Mutations that can be harmful, if they are passed on to the baby. Oocyte donors having such mutations are not included in our program.
In vitro Fertilization (IVF)
In Vitro Fertilization (IVF)
IVF achieves pregnancy by fertilizing the woman’s eggs (oocytes) outside her body. The oocytes are obtained under mild anaesthesia using transvaginal guided Sonographic procedures.
ISOLATION OF OOCYTES
The semen is processed in order to obtain the most healthy sperm. The eggs and sperm are incubated together. Next day, the oocyte is checked for fertilization. The embryos are ready for transfer subsequently. IVF is ideal for couples with tubal blockage or pelvic adhesions, previous tubal sterilization not amenable to reversal and some cases of endometriosis and pelvic factor.
PESA/TESA/TESE/Micro TESA
Percutaneous Epididymal Sperm Aspiration (PESA), Testicular Sperm Aspiration (TESA) and Testicular Sperm Extraction (TESE) are used when there is no sperm in the ejaculate (azoospermia). This can result from an obstruction in the reproductive tract (obstructive azoospermia) or due to lack of sperm production (non obstructive azoospermia). Obstructive azoospermia may be due to failure of the sperm passages to develop, blockage of the tubes transporting the sperm (due to infection or due to surgical blockage of the Vas following male sterilization).
We also perform Micro TESA in men with severe oligospermia or Non-obstructive Azoospermia. This technique is done under an operating microscope.The remaining sperm and testicular tissue can be frozen for future attempts.
We offer surgical sperm retrieval techniques for men who suffer from azoospermia (no sperm in the ejaculate). Percutaneous Epididymal Sperm Aspiration (PESA) / Testicular Sperm Aspiration (TESA) / Testicular Sperm Extraction (TESE), are procedures in which sperm from men with azoospermia can be removed from the epididymis or the testis and used to fertilize eggs by ICSI. These techniques are utilized in conditions of obstructive and non-obstructive azoospermia.
We have one of the largest series in India using testicular and epididymal sperm for ICSI.
The Jaslok team had the first pregnancy in India using testicular sperm and ICSI. (Parikh et al., Indian Journal of Urology 1997; 13: 97-98.)
Laser Assisted Hatching (LAH)
 Prior to implantation, the embryo has to escape out of its protective shell known as the zona pellucida by a process known as Hatching. If this process is not completed properly, implantation will fail and pregnancy will not occur. We offer Laser technology for Assisted Hatching (LAH), where a laser beam is focused over the zona pellucida making a small opening, between 30-40 microns to facilitate embryo hatching. This technique is particularly useful in women where the embryo has a thick zona and also in women with previous failed cycles and older women where the zona tends to be thick. Laser Assisted Hatching is also carried out during the process of Preimplantation Genetic Testing (PGT) where the diode laser is also used to make an opening in the zona through which a cluster of 5-8 cells called Trophectoderm cells are aspirated at the time of PGT. However, selection of this procedure for the right application is important.
Prior to implantation, the embryo has to escape out of its protective shell known as the zona pellucida by a process known as Hatching. If this process is not completed properly, implantation will fail and pregnancy will not occur. We offer Laser technology for Assisted Hatching (LAH), where a laser beam is focused over the zona pellucida making a small opening, between 30-40 microns to facilitate embryo hatching. This technique is particularly useful in women where the embryo has a thick zona and also in women with previous failed cycles and older women where the zona tends to be thick. Laser Assisted Hatching is also carried out during the process of Preimplantation Genetic Testing (PGT) where the diode laser is also used to make an opening in the zona through which a cluster of 5-8 cells called Trophectoderm cells are aspirated at the time of PGT. However, selection of this procedure for the right application is important.
 Our centre utilizes the Diode laser which is the safest laser in ART. The entire procedure is completed in a few milliseconds.
Our centre utilizes the Diode laser which is the safest laser in ART. The entire procedure is completed in a few milliseconds.
We achieved the first pregnancy in India using this technique combined with blastocyst transfer (LAHBT). This facilitates its hatching in the uterus and improves implantation and pregnancy rates. In our published study we have demonstrated pregnancy rates to rise from 19% to 44% in this group of women. (Parikh et al., Indian Journal of Obstetrics and Gynacology. November, 2000.)
Preimplantation Genetic Testing (PGT)
What is Preimplantation Genetic Diagnosis (PGD) / Preimplantation Genetic Screening (PGS) / Preimplantation Genetic Testing (PGT) / Preimplantation Genetic Testing for Aneuploidy (PGT-A) / Preimplantation Genetic Testing for Structural Rearrangements (PGT-SR) / Preimplantation Genetic Testing for Monogenic Disorders (PGT-M)?
The terms Preimplantation Genetic Diagnosis (PGD) and Preimplantation Genetic Screening (PGS) have broadly been replaced by Preimplantation Genetic Testing (PGT), with different sub-categories. PGT is an early form of prenatal diagnosis. During the ICSI cycle, a few cells called Trophoblast cells are removed (biopsied) from the Day 5 / Day 6 embryo and are checked for genetic abnormalities. The embryos, that are reported normal, are then selected for embryo transfer.
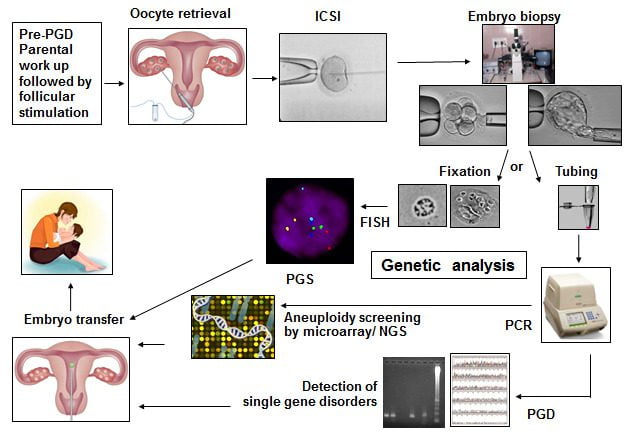
Preimplantation Genetic Screening (PGS) also called Preimplantation Genetic Testing for Aneuploidy (PGT-A) is to check for the number of chromosomes and any major gain or loss of genetic material in the chromosome.
What are chromosomes?
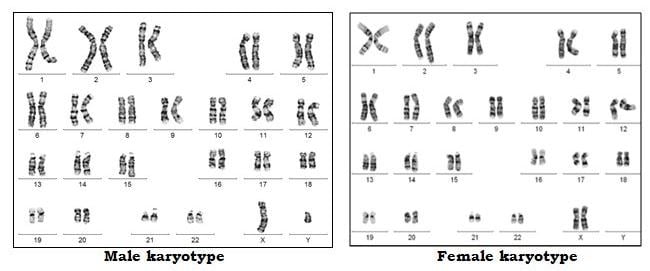
Chromosomes contain the genetic material of the cell. They are present in the nucleus of the cell. Chromosomes comprise molecules of DNA. Our DNA is organized into small structures called genes. These influence our growth and development. Genes are transmitted from parent to offspring as they are considered to be the basic units of inheritance.
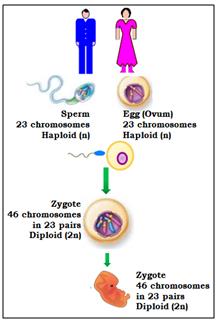
Forty-six chromosomes are present in all human cells, except the gonadal cells (eggs and sperm). The egg and the sperm each have 23 chromosomes out of which 22 are autosomes and 1 is the sex chromosome. In the egg, the sex chromosome is always X. In each sperm, the sex chromosome can be either X or Y. When the egg and sperm unite at fertilization, the total number of chromosomes becomes 46. Depending on which sperm has fertilized the egg, the embryo becomes either male (XY) or female (XX).
In each embryo, chromosomes 1 to 22 are present in 2 copies where one comes from the sperm (biological father) and one comes from the egg (biological mother). Thus, an embryo with 46 chromosomes (22 pairs of autosomes and 1 pair of sex chromosomes) is considered euploid (Normal) embryo.
When the number of chromosomes alters, the embryo is aneuploid (Abnormal). Monosomy occurs when there is loss of 1 copy of the chromosome from the pair. When there is an extra copy present, it is called trisomy of that particular chromosome. When the entire set of 23 chromosomes is extra, it is called triploidy. When both the sets of 23 chromosomes are extra, it is called tetraploidy. If the copy number is more than 4, then it is called polyploidy. If only 1 set of chromosomes (23) is present then it is called haploidy. If more than one cell line is present where one is with 46 chromosomes and other with more or less than 46, it is called as mosaicism. Mosaicism can occur during embryonic division at any stage.
Aneuploidies of embryos can result in spontaneous miscarriages, stillbirths, or developmental disorders in the newborn. The most common aneuploidies are of chromosomes 13, 18, 21, X and Y, followed by aneuploidies for chromosomes 16 and 22.
A chromosome translocation is a chromosome abnormality due to rearrangement of parts of 2 different chromosomes. Translocations can be balanced (an exchange of material with no extra or missing genetic information) or unbalanced (where the exchange of chromosome material results in extra or missing genes and normal functionality is affected). The couples with balanced translocation have a 25% chance of having a child with a normal chromosomal make-up and a 25% chance of having a child with balanced translocation (they are fully functional). There is a 50% risk of having a child with unbalanced translocation which may lead to miscarriage, Intrauterine growth restriction (IUGR), Intrauterine fetal death (IUFD) or delayed developmental milestones.
What is a Gene disorder?
Just like chromosomes come in pairs, most genes also come in pairs, one from the egg and one from the sperm. When, the function of a gene is altered by a change (called a mutation) in the specific sequence of the DNA, a genetic disease results. These mutations can be transmitted in families from generation to generation (inherited), or can be a new change in an individual (de novo). These mutations lead to either single gene or multiple gene disorders.
Genetic diseases can be inherited in different ways. A dominant genetic disease is caused by a mutation in one copy of a gene. The risk for a parent with the disease to pass the condition to the child is 50%. e.g. Huntingtons Chorea.
A recessive genetic disease is caused by a mutation in both copies of a gene. A carrier has one normal copy of the gene and one copy with a mutation. Most of them are healthy since having one normal copy is enough to prevent the disease. Two carrier parents have a 25% risk of having a child with the genetic disease. e.g. Thalassemia, Cystic Fibrosis.
Sex-linked genetic diseases are caused by mutations on the X or Y chromosomes. Sex-linked diseases can be dominant or recessive and affect males and females differently. In some genetic diseases, the abnormality in the gene keeps increasing in the next generation and its expression is seen only when an affected child is born. e.g. Fragile X Syndrome. Hence, if there is any history of having an affected child, it is advisable to undergo testing for the next pregnancy and also test the other family members.
Preimplantation Genetic Testing is carried out for couples who are at a high risk for various genetic disorders, those with male factor, in advanced maternal age or repeated failed IVF. PGT-M is also done for genetic conditions such as Thalassemia, Sickle Cell Disease, Duchenne Muscular Dystrophy, Leigh Syndrome, Huntington Disease, Neurofibromatosis, Cystic Fibrosis and in Hereditary Cancer Predisposition Syndromes.
Steps involved in the PGT cycle:
For PGT testing, multiple embryos are developed by performing ICSI. The steps involved in PGT are:
- Pre-PGT work-up:
For the couple opting for PGT for single gene disorders (PGT-M), it is important to carry out Genetic testing for the disorder in both the partners prior to PGT-M. This determines the mutation (alteration in genetic material) status of both partners. Based on the genetic report, the actual PGT protocol is developed. PGT for single gene disorders (PGD/PGT-M) is carried out only for the known disorder and mutation which is identified for the couple. Other unknown genetic abnormalities cannot be tested at this time.
Other important genetic tests for PGT:
1. Karyotyping: Chromosomal analysis is carried out to check for any aneuploidy or translocation or presence of mosaicism.
2. Sperm FISH to check for a percentage of aneuploid sperm. (i.e. sperm with an abnormal number of chromosomes).
3. Sperm DNA Fragmentation Indiex to check for the percentage of sperm with DNA fragmentation.Many routine blood parameters are also tested. Once these reports are ready, the ovarian stimulation cycle begins.
- Hormonal stimulation and oocyte retrieval:The female partner undergoes ovarian stimulation using hormones in order to obtain adequate number of eggs. The eggs are collected under anesthesia.
- ICSI/ IMSI: ICSI (Intracytoplasmic sperm injection) is performed. A single sperm is injected into the cytoplasm of the mature egg. After fertilization, the embryo is further incubated in culture till Day 5/Day 6/Day 7.

- Embryo biopsy at the Blastocyst stage:For chromosomal aneuploidy screening (PGT-A), embryo biopsy is generally performed on Day 5 or Day 6 (Blastocyst stage). For single gene disorders (PGT-M) the embryo biopsy is performed on Day 5 or Day 6 (Blastocyst Stage).
The Blastocyst is made up of a cluster of cells called the Inner Cell Mass (ICM): which forms the fetus and the trophectoderm cells (which form the placenta). By gentle micromanipulation about 5-8 trophectoderm cells are aspirated into a pipette, loaded into the tube and sent for genetic analysis.
All of the above steps are carried out in the Embryology laboratory. The trophectoderm cells are then transported to the Genetics Lab for further testing. After biopsy, the embryos are cryopreserved till the day of embryo transfer.
- Different techniques of genetic testing include Fluorescence in Situ Hybridization (FISH), Array Comparative Genomic Hybridization (aCGH) and Next Generation Sequencing (NGS).
- Frozen Embryo Transfer (FET) of the genetically normal embryos is carried out and sometimes in the same cycle by doing a Quick Run Analysis in a subsequent cycle.
- A pregnancy test is performed 14 days later.
Please note that our centre does not perform any form of sex determination by PGT in any form.
Objectives of PGT:
- Prevention of chromosomally abnormal births for couples having a history of failed fertilization after IVF-ICSI, missed abortions or for carriers of balanced translocations
- To increase the chances of implantation
- Reducing the incidence of spontaneous miscarriages
- Reduction in multiple offspring
- Eliminates the risk of being affected with a familial gene disorder.
- Savior sibling (HLA matched) to cure a previously affected sibling with cord blood stem cells.
Risk and Limitations:
Even though the benefits of PGT-M/PGT-A are considerable, some risks and limitations have to be kept it mind. This procedure cannot guarantee a healthy pregnancy or eliminate the risk of miscarriage, stillbirth or the birth of a child with an abnormality.
- If less number of oocytes are retrieved, the ICSI cycle may have to be repeated.
- Occasionally, there may be decreased fertilization, few healthy embryos or embryos that cleave slowly and do not reach the Blastocyst stage. Hence a biopsy may not be possible.
- If the quality of the embryo is poor, the genetic material present in the biopsied cells may be degraded causing breaks in the cellular DNA. Such DNA material may not give results. In such a case, a repeat trophectoderm biopsy may be needed.
- It is possible that all the embryos may show genetic abnormalities and hence embryo transfer may not be possible.
Blastocyst Culture
 A blastocyst is an embryo that consists of more than 100 cells and forms 5 days after fertilization. Using sophisticated culture media, we can select embryos that remain healthy in culture and reach the blastocyst stage prior to transfer. A higher implantation rate has been observed following transfer of blastocysts. Currently our practice is to transfer 1 to 2 embryos at blastocyst stage. The extra embryos are then frozen by vitrification. In case of less number of good quality embryos, a Day 3 transfer of 2 embryos can be performed.
A blastocyst is an embryo that consists of more than 100 cells and forms 5 days after fertilization. Using sophisticated culture media, we can select embryos that remain healthy in culture and reach the blastocyst stage prior to transfer. A higher implantation rate has been observed following transfer of blastocysts. Currently our practice is to transfer 1 to 2 embryos at blastocyst stage. The extra embryos are then frozen by vitrification. In case of less number of good quality embryos, a Day 3 transfer of 2 embryos can be performed.
We achieved the first pregnancy in India using the technique of Laser Assisted Hatching and Blastocyst Transfer (LAHBT) in 2000.
Embryo / Oocyte / Sperm Cryopreservation
 Treatment with ovulation induction at the time of Assisted Reproduction produces many oocytes. We cryopreserve extra embryos for future cycles. Our freezing protocol is by vitrification. All embryos are coded, labeled and stored in special containers to maintain their identity. We have an active oocyte vitrification program. Oocyte cryopreservation is carried out prior to chemotherapy and in women who want to delay childbearing.
Treatment with ovulation induction at the time of Assisted Reproduction produces many oocytes. We cryopreserve extra embryos for future cycles. Our freezing protocol is by vitrification. All embryos are coded, labeled and stored in special containers to maintain their identity. We have an active oocyte vitrification program. Oocyte cryopreservation is carried out prior to chemotherapy and in women who want to delay childbearing.
The process of Vitrification and the process of thawing has to be very precise to ensure survival of the oocytes and embryos.
Our IVF Centre has installed the Cryogatt Freezing System which is a Safety net for freezing Embryos, Sperm and eggs using Artificial Intelligence & Radiofrequency Identification so that there is no mixup of samples.
We are the first IVF Centre in Asia to have installed this system.
All of these biological materials are stored at -196 degrees Centigrade and can be indefinitely stored. The samples are placed in straws or vials that are either bar coded or labelled manually. One has to be very careful to avoid mix up of biological samples. Sometimes the labels can become unsticky. In such cases identification of the biological sample can become difficult. Also the correctly labelled straw has to be identified and removed from the other samples in less than 15 seconds. Any longer exposure can damage the biological material. The Cryogatt System bypasses all these difficulties.
How does the Cryogatt System work?
The Cryogatt System has Radiofrequency Identification that is safe for humans.
Each straw is tagged with a RF Identifier (RFID). A platform containing the software program is placed on the rim of the cryostorage tank. As the straw is being removed, the program’s tagging system identifies the straw and the Reader displays the patient’s name ensuring the right sample is being thawed. This also prevents inadvertent thawing of other samples thereby preventing any human error.

There are instances reported where a mixup of Embryos and Sperm have resulted in the birth of biologically unrelated babies. Our system ensures that this human error does not occur.
We currently have more than 20000 eggs and embryos in storage. Our Sperm bank has 6500 samples stored including 1200 samples of semen stored for men undergoing chemotherapy.
Each container of up to 100 vials or straws can be read in seconds. This can even take place within a freezer or the vapour phase of a Dewar without the need for samples to be individually handled for identification, thus protecting the material from dangerous temperature rise.
Andrology Laboratory
Semen Analysis
A detailed semen analysis is important for assessing Male Factor Infertility. We follow the WHO 2010 & 2021 guidelines for Semen Analysis. A detailed examination of the concentration, motility and morphology of sperm is carried out along with biochemical examination of the semen. Emphasis is put on examination for pus cells and immature sperm cells.
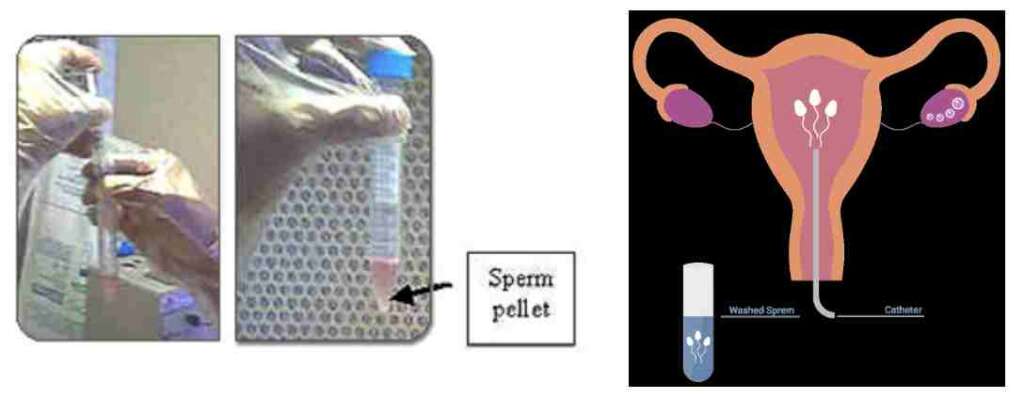
Sperm Processing for Intrauterine Insemination (IUI)
The semen sample is processed by washing, centrifugation and migration. A suitable culture medium is used. The final migration step is done under stringent culture conditions using CO2 incubation. The spermatozoa thus obtained are free of debris and bacteria, are energetic and improve the success rates of intrauterine insemination. We have 2 special rooms with privacy for semen collection. Women with ovulatory dysfunction, previously treated endometriosis with patent tubes, luteal phase defects, cervical factor infertility and polycystic ovarian disease can try IUI first before trying more advanced techniques of Assisted Reproduction. Men with slightly compromised semen parameters may benefit from IUI.
For more information click here

Semen Cryopreservation and Banking
We routinely have a back-up semen sample frozen for couples who are undergoing treatment in case of inability to give a semen sample on the day of the IUI, IVF or ICSI. Men undergoing orchidectomy/cancer treatment prior to radiation and chemotherapy can also avail of our cryopreservation facilities. Those undergoing ICSI are encouraged to freeze their sperm so that we have adequate number of sperm at the time of ICSI. We routinely cryopreserve testicular and epididymal sperm, so that repeated attempts at obtaining fresh epididymal and testicular sperm are avoided.
Donor Insemination Program
We follow all the guidelines of the ART Act of India and the Indian Council of Medical Research (ICMR). We have a tie up with a sperm bank for donor semen. This has undergone stringent evaluation. The donors are tested for HIV, Hepatitis B and C and VDRL. The semen quality is ensured by following the WHO criteria. Karyotyping of the Semen Donor is carried out. Specific Genetic Tests can be performed if requested by the couple.
Clinic for Recurrent Miscarriages and Pregnancy Loss
Recurrent pregnancy loss can be caused by endocrine, immunological, anatomical and genetic factors. Our clinic offers genetic counseling as well as immunomodulation for this condition. We have one of the highest pregnancy rates and live birth rates for women with recurrent pregnancy loss.
What is Immunomodulation?
A small group of women are prone to repeated miscarriages due to an immunological problem. This can be detected by a test called Immunophenotyping. The test is performed on the endometrial tissue obtained by means of a simple procedure called endometrial biopsy. If the woman has an immunological cause for miscarriage, intravenous therapy with Immunoglobulins is given over a period of time.
Some women develop autoantibodies to their pregnancy resulting in repeated pregnancy loss. We have one of the largest series in the world documenting the role of Ovarian Antibodies in causing recurrent miscarriages.
Repeated miscarriage may be caused by sharing of HLA (Human Leucocyte Antigen) between the couple. We have successfully treated such couples to have healthy babies.
Genital Tuberculosis:
This is a major factor for female infertility in India. We include a molecular marker test for the TB bacterium using immunocytochemistry along with other tests. This has helped us to detect TB at a very early stage of the disease (latent stage) thereby improving the prognosis of genital TB if detected early.
We also offer our patients TB-PCR, a diagnostic test to rule out the involvement of TB as a causative agent for infertility. The test involves obtaining a small amount of tissue from the lining of the uterus since the uterus is a common site for tuberculous involvement, followed by analysis of the sample by Polymerase Chain Reaction (PCR), which is a DNA or RNA based diagnostic tool. This test is specifically recommended for patients with repeated failed ART cycles in the presence of a thin uterine lining. A test called Gene Expert is also carried out to check which antibiotics the TB germ is susceptible to. Culture for TB bacteria is also done.
We have scientific documentation of the high incidence of genital TB in Indian women with pelvic factor infertility (Fertility and Sterility, Vol. 67, No.3, 1997, 497-500). The data consists of over 1500 women diagnosed with Genital TB.
Endoscopic surgery
Our team of doctors have extensive experience in Laparoscopy and Hysteroscopy procedures. We constantly update ourselves with the latest surgical techniques and are equipped with state-of-the-art surgical instruments. Our team is led by Dr. Neeta Warty who is internationally acclaimed.
Laparoscopy is a procedure that allows the doctor to look directly at the uterus, fallopian tubes, ovaries, appendix, and other organs through keyhole surgery. Operative laparoscopy enables the surgeon to correct defects and remove tumors & cysts in the uterus, fallopian tubes and ovaries without the patient undergoing an open surgery. This technique is known as minimally invasive surgery and involves minimal discomfort to the patient. This technique is helpful in the presence of adhesions, endometriosis, pelvic tuberculosis, fibroids, ovarian tumors and cysts and in intestinal conditions such as appendicitis.
Our team has one of the largest experiences in the country with operative laparoscopy.
Hysteroscopy is a procedure, which allows the doctor to visualize the uterine cavity from within. Operative Hysteroscopy enables the surgeon to correct defects within the uterine cavity. This technique is very helpful to treat intrauterine septum, adhesions and intracavity fibroids.
Our team has one of the largest experiences in the country with operative hysteroscopy.
Dr Neeta Warty is a pioneer in the field of endoscopy and has performed more than 8000 laparoscopies and hysteroscopies at our IVF centre.
Counseling
 Infertility and its treatment cause significant stress to the couple. This is detrimental to the reproductive cycle. Before definitive treatment begins, we encourage couples to talk about their stress factors and initiate counseling. We have one of the largest series on psychiatric screening and counseling for ART and many other studies cite our work in this field. Dr. Rajesh Parikh, Consultant Neuropsychiatrist and his team of counsellers will guide you through your cycle.
Infertility and its treatment cause significant stress to the couple. This is detrimental to the reproductive cycle. Before definitive treatment begins, we encourage couples to talk about their stress factors and initiate counseling. We have one of the largest series on psychiatric screening and counseling for ART and many other studies cite our work in this field. Dr. Rajesh Parikh, Consultant Neuropsychiatrist and his team of counsellers will guide you through your cycle.
We have counselled more than 10000 infertile couples.
Intrauterine Insemination (IUI)
Intrauterine Insemination: IUI
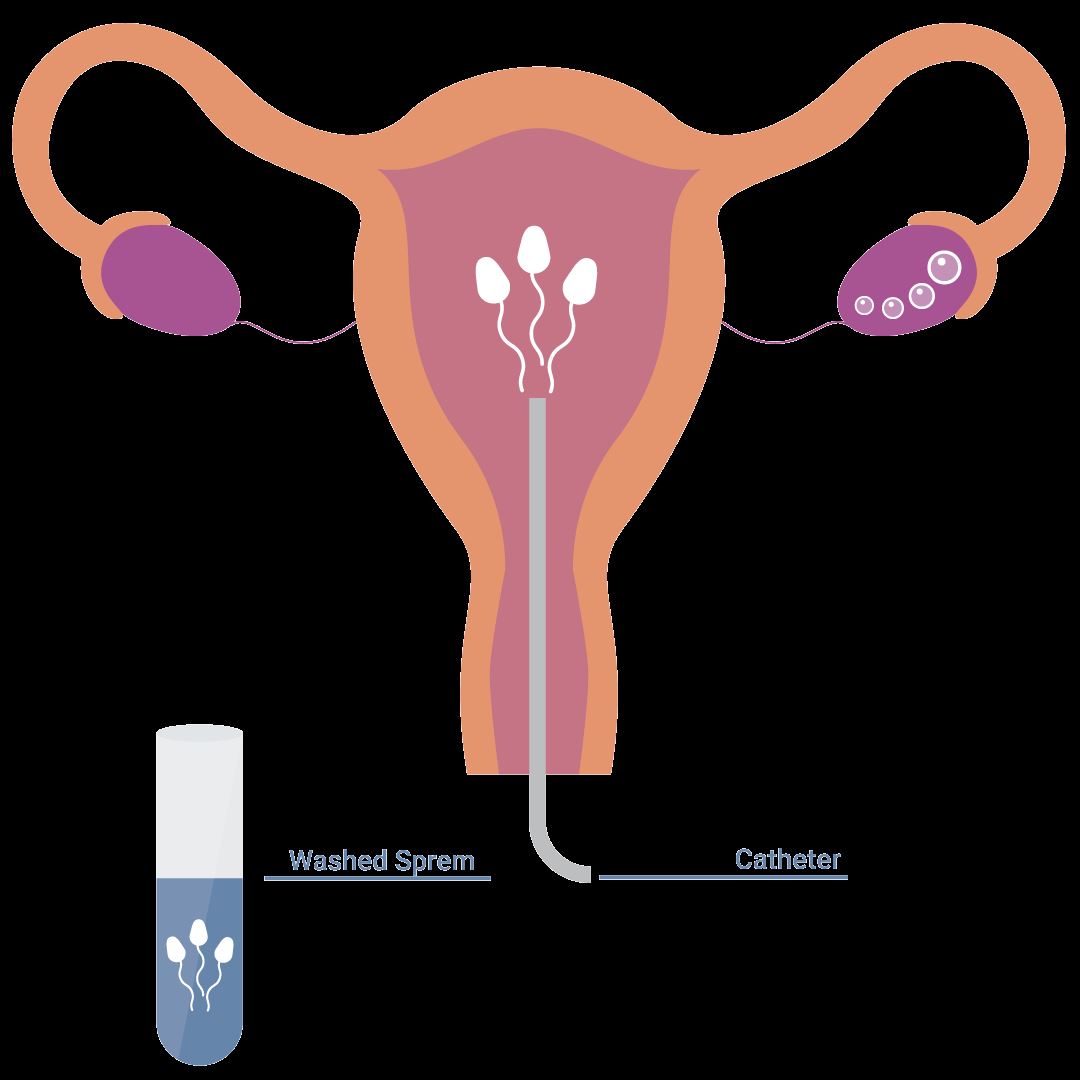
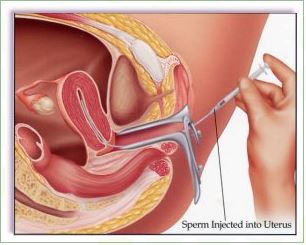
Intrauterine Insemination (IUI) is a fertility enhancing procedure in which the ejaculated semen is washed free of seminal plasma and other debris, concentrated into a small volume of highly motile sperm, and is then injected into the uterus as close to the fallopian tubes as possible, in an attempt to achieve a pregnancy. The purpose of IUI is to increase the number of sperm that reach the fallopian tubes and thus increase the chances of fertilization. IUI provides the sperm an advantage by giving it a head start but still requires the sperm to reach and fertilize the egg on its own. It is a less invasive and less expensive option compared to In Vitro Fertilization.
When is IUI used?
Male Factor Indications
- Marginally low sperm count
- Decreased sperm motility
- Increased number of abnormal sperm (abnormal morphology)
- Disorders of sperm function, such as increased sperm DNA fragmentation
- Defects of the penis, e.g., hypospadias or severe penile curvature
- Retrograde ejaculation or other forms of ejaculatory dysfunction, e.g. patients with spinal cord injury needing electro-ejaculation
- Sexual dysfunction, premature ejaculation
Female Indications
- Poor cervical mucous with a poor post coital test
- Persistent cervicitis
- Cervical stenosis
- Endometriosis
- Ovulatory dysfunction
- Psychological or physiological sexual dysfunction
Common Indications
- Unexplained infertility
Indications for Donor IUI
- Azoospermia with spermatogenic failure
- Previous vasectomy not amenable to IVF/ICSI /TESA/PESA
- Previous cancer therapy with radiation or chemotherapy.
Contraindications for IUI
- Severe male infertility
- Tubal Disease
- Moderate to severe endometriosis
Fertility Drugs for Intrauterine Insemination (IUI)
- Clomiphene Citrate Letrozole
- Gonadotropins
- IUI can be done in a natural cycle also
How does IUI work?
Before intrauterine insemination, ovulation-stimulating medication can be used, in which case ultrasound monitoring will be necessary to determine when the eggs are mature. The IUI procedure is performed around the time of ovulation, typically about 24-36 hours after the surge in LH hormone that indicates ovulation will occur soon or 36 hours after the hCG trigger.
The semen sample is washed in the lab to separate the semen from the seminal fluid. A catheter is used to insert the sperm directly into the uterus. This process maximizes the number of sperm that are placed in the uterus, thus increasing the possibility of conception. There are different techniques of sperm preparation such as sperm Migration and Swim up, Overlay, Double Gradient Centrifugation (Dgc), MACs, Microfluidics technology.
The IUI procedure takes a few minutes and involves minimal discomfort. A pregnancy test is done 14 days after the IUI.
Success Rates with IUI
Success rates with IUI depend upon the procedure being performed
- For the correct indications
- Avoiding the performance of IUI when contraindications exist
- The age of the woman
- Sperm Parameters
By and large, birth rates per cycle of IUI performed for the correct indications are reported to be about
- 15% for women under the age of 30
- 12% for women ages 30 to 35
- 7% to 8% for women ages 35 to 39
- Less than 2% for women over the age of 40.
Intra Ovarian Platelet Rich Plasma (IOPRP) Instillation
We are seeing an alarming trend of declining AMH and Diminished Ovarian Reserve in Indian women who are seeking infertility treatment. The decline is more noticeable in women in the age range of 30-37 years. This declining trend is creating a generation of young Indian women with Poor Ovarian Reserve (POR), Diminished Ovarian Reserve (DOR), Premature Ovarian Insufficiency (POI) and therefore diminished reproductive potential. In spite of trying various methods of ovarian stimulation, these young women do not respond well to ovarian stimulation and may produce no eggs. For most young women, accepting oocyte donation is not an option. In our experience, use of the Platelet Rich Plasma has shown beneficial effects in Ovarian Rejuvenation. We have introduced a simplified technique of using Intra Ovarian Platelet Rich Plasma (IOPRP) which has shown encouraging results. We have achieved a 44% pregnancy rate in women with poor ovarian reserve after 3 cycles of PRP as compared to 22% in women who had poor ovarian reserve and did not do IOPRP (Parikh et al. 2022).
What does PRP contain?
Platelets are cytoplasmic fragments of megakaryocytes which contain numerous proteins, several growth factors and cytokines stored in the cytoplasmic granules. ADP, ATP, Ca+, Histamine, Serotonin and Dopamine present in the dense granules play an important role in tissue homeostasis. More than 30 bioactive proteins are stored in the alpha granules. Some of these assist in wound healing. Fibrin, Fibronectin, Vitronectin and sphingosine-1-phosphate act as Adhesion molecules. Some of the Growth Factors secreted are the Platelet Derived Growth Factor (PDGF), Vascular Endothelial Growth Factor (VEGF), Transforming Growth Factor beta (TGF-β), Connective Tissue Growth Factor (CTGF), Keratinocyte Growth Factor (KGF), Basic Fibroblast Growth Factor (bFGF), Epidermal Growth Factor (EGF), Insulin-like Growth Factor 1 (IGF-1) & 2 (IGF-2) & Hepatocyte Growth Factor (HGF).
What is the function of Platelet secreted growth factors?
Platelet secreted growth factors act on cell proliferation and neoendothelial cell generation. They have rejuvenating properties. These factors are responsible for cell migration and differentiation, cell proliferation, activation of angiogenesis and tissue regeneration, tissue necrosis resolution and chemotaxis.
What are Alpha granules:
Alpha granules (α-granules) are a component of platelets. The term “alpha granules” is used to describe granules containing several growth factors such as IGF-1, PDGF, TGF-β and other clotting proteins.
What are Dense Bodies:
Dense Bodies (also known as dense granules or delta granules) are specialized secretory organelles. Dense granules are found only in platelets and are smaller than α-granules. The dense granules of human platelets contain substances which are necessary for several steps of coagulation.
How does PRP work:
Platelets promote healing at the site of injury. PRP promotes wound healing by tissue necrosis resolution, chemotaxis and attracts stem cells, cell regeneration, cell proliferation, extra cellular matrix synthesis, remodelling, angiogenesis and epithelialisation. They also have a positive impact on promoting follicle formation, matrix and collagen formation, activation of angiogenesis thus helping in healing, angiogenesis and regeneration. The growth factors in the cells surrounding the eggs control angiogenesis and hence, these activate angiogenesis. The secretory proteins such as PGDF and TGF-β bind to the target cells such as mesenchymal stem cells, osteoblasts, fibroblasts, endothelial and epidermal cells, promoting cellular proliferation, migration, differentiation and osteoid formation. PRP treatment for the ovaries enhances ovarian regeneration and reactivates folliculogenesis.
How is PRP prepared?
Blood is collected from the woman and centrifuged at different speeds to get platelet rich plasma. This amount is mixed with a solution after which it is ready for injection.
How is it injected?
For Ovarian rejuvenation, PRP is injected into the outer surface of the ovary. The procedure is carried out under mild anaesthesia using transvaginal ultrasound. About 1.5ml is injected in each ovary.
Does IOPRP have any side effects?
No. It does not as the PRP is prepared from the woman’s own blood (Autologus).
Does IOPRP injection cause cancer?
No. The growth factors in PRP do not cause granulosa cell tumors or any other form of cancer unlike the risk of granulosa cell tumors after injecting Bone Marrow Derived Stem Cells.
Our Success
The clinical pregnancy rate per transfer was 46.88% per embryo transfer in women who underwent IOPRP versus 25% per embryo transfer in women who did not opt for IOPRP (Parikh et al., 2022).
Intra Uterine Platelet Rich Plasma (IUPRP) Therapy
Intrauterine PRP:
In India, Tuberculosis is a major health problem. The burden of Genital TB is underestimated as most of the patients are asymptomatic. Endometrial TB impairs the lining and decreases the implantation rates. It causes implantation failure by immune modulation of the endometrium and by making the uterine lining non receptive to the embryos.
We have innovated the simplified technique of intrauterine instillation of homologous Platelet Rich Plasma.
Our study has shown a 24% pregnancy rate in women with a history of genital TB or thin lining who had not conceived before. It is also very promising in women who have never conceived before due to endometrial lining issues. Our study also demonstrated a decrease in the rates of ectopic pregnancy following intrauterine instillation of PRP thus explaining its major role in making the uterine milieu conducive to implantation and pregnancy.
How is PRP prepared?
Blood is collected from the woman and centrifuged at different speeds to get platelet rich plasma. This amount is mixed with a solution after which it is ready for injection.
How is it instilled?
PRP is instilled into the uterine cavity using an intrauterine catheter using sterile precautions.
When is it instilled?
PRP is instilled three times during the cycle and is completed 48 hours before the embryo transfer.
How long does the effect of PRP last?
For the IUPRP, we believe that the effect is restricted to the cycle in which it is done.
Are the results seen immediately……Same cycle?
Yes.The effects are reflected in the endometrial lining in the same cycle .The positive outcome of pregnancy is the marker of the success.
Does IUPRP have any side effects?
No. It does not because PRP is prepared from the women’s own blood (Autologous).
Does IUPRP injection cause cancer?
No. The growth factors in PRP do not cause cancer.
Who can benefit from PRP?
- Women with failed cycles due to persistent thin lining.
- Women not responding to conventional modes for improving the endometrium (Sildenafil/Aspirin/ increased doses of Estradiol Valerate /Intrauterine instillation of GCSF)
- Women with history of miscarriages due to endometrial issues.
- Women with history of Ectopic pregnancy.
- Women with treated genital TB who have not conceived before.
Intrauterine PRP should not be used when there is
- Active infection of the endometrial cavity.
- A high level of CD138 positive cells in the endometrium. This is suggestive of chronic inflammation which should be treated first.
- Presence of acute pelvic infection or Pelvic Inflammatory Disease (PID).
Our work on Intrauterine PRP has been presented at the American Society for Reproduction Conference in Philadelphia,USA.
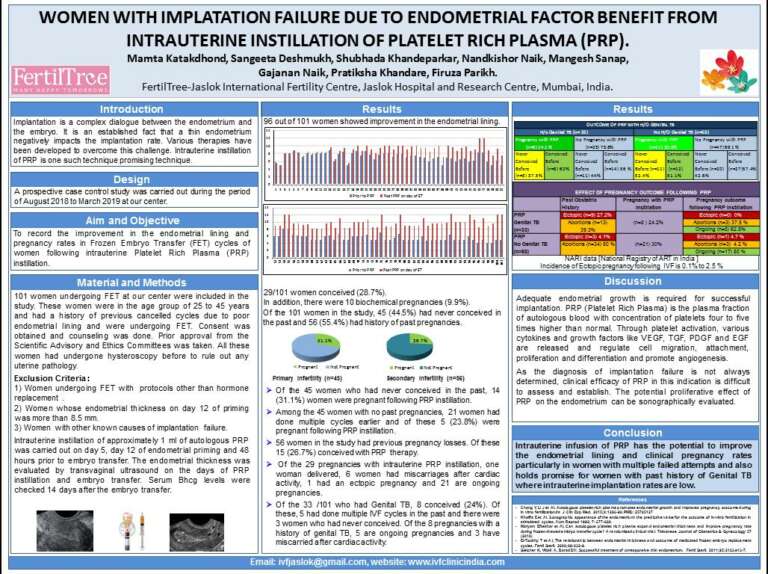
A poster presentation at American Society for Reproductive Medicine conference, October 2019 at Philadelphia, USA.
Mamta Katakdhond, Sangeeta Deshmukh, Shubhada Khandeparkar, Nandkishor Naik, Mangesh Sanap, Gajanan Naik, Pratiksha Khandare, Firuza Parikh. Women with Previous failed IVF benefit from Intrauterine instillation of Platelet Rich Plasma. ASRM 2019. Fertil Steril 2019:112(3), supl e185 P197.